Compliance with the 21 CFR Part 11 standard
21 CFR Part 11 is the part of Title 21 of the Code of Federal Regulations that establishes the United States Food and Drug Administration (FDA) regulations on electronic records and electronic signatures.
21 CFR Part 11 describes the requirements for creating, modifying, maintaining, archiving, and retrieving electronic records.
Configuration Utility improvement
- Configuration Utility provides a possibility to enable and disable plugins, devices, and tags.
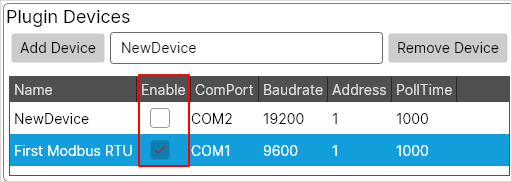
- Now, the user can create a new configuration file and set it as a default configuration. When starting, KPA Automation Server and Configuration Utility load the default configuration from the INI file.
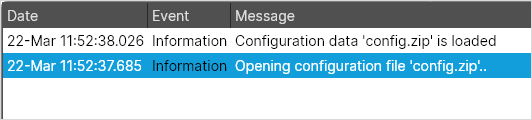
- The traces component is implemented in Configuration Utility. Trace events includes an event date and a message.
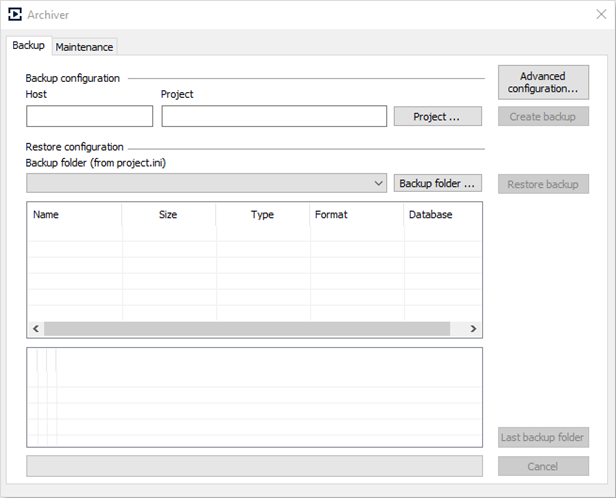
Implementation of Archiver
The Archiver was developed to create backups of user projects and to collect information from the logs of both the Windows OS and KPA Automation components.